
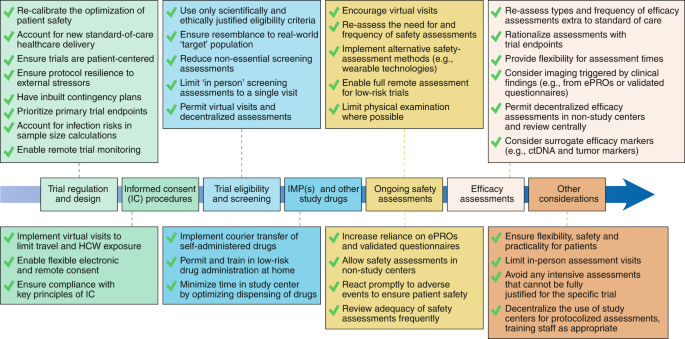
Furthermore, the recent termination of future BCG production at Sanofi Pasteur threaten global supply of BCG. Patients who fail BCG then require either surgical removal of the bladder with urinary diversion or chemotherapy and radiation, both of which have considerable morbidity. However, it is known that about 50% of patients fail BCG, significantly increasing the risk of progression and death.

Intravesical BCG is the standard of care for patients with intermediate to high risk NMIBC. The current standard of treatment for NMIBC is Transurethral Resection of Bladder Tumor (TURBT) with or without intravesical therapy, such as Mitomycin C (MMC) or Bacillus Calmette-Guerin (BCG), determined by the stage and grade of tumor. The high rate of recurrence with current therapies requires lifelong active surveillance, making bladder cancer the most expensive cancer to treat from diagnosis to death. While NMIBC is associated with a higher than 88 percent survival rate over 5 years, up to 70 percent of NMIBC recur after initial treatment, with 10–20 percent progressing to muscle-invasive bladder cancer. More efforts would be needed to identify an optimal measurable endpoint and establish a better combination of pharmacological and nonpharmacological interventions to improve the functional prognosis for patients with cancer cachexia.Ĭancer cachexia disability endpoint functional prognosis patient-centered outcome.Urothelial carcinoma of the bladder is the fourth most common malignancy in men, with about 70% being non-muscle invasive bladder cancer (NMIBC). It might be also a suitable patient-centered outcome responsive to multiple physical changes in cancer cachexia, and patients might find it more acceptable than other classical endpoints. Disability-free survival has recently been used as a functional endpoint in clinical trials in several research fields. Nonpharmacological interventions have been widely tested, and functional endpoints have increasingly been selected in combination with standard endpoints of body mass or lean body mass.

Compared with trials before the CR, trials after the report revealed that the study populations tended to be at the earlier stage of cachexia and included patients with precachexia or those at risk for cachexia. This review aimed to summarize designs and endpoints of 65 randomized controlled trials for cancer cachexia in the past 16 years and seek clinically relevant patient-centered outcomes for future clinical trials.
This may be partially due to the lack of a widely accepted common endpoint for clinical trials. However, no standard treatment has been established so far. The diagnostic and staging criteria for cancer-associated cachexia have been established through an international consensus report (CR) published in 2011, which may greatly influence the designs and interventions of future clinical trials. Cachexia is an old disease but a new research area that has recently been vigorously investigated.


 0 kommentar(er)
0 kommentar(er)
